A Weather Instrument Used To Measure Changes In Air Pressure?

A barometer is a scientific instrument that is used to measure air pressure level in a certain environment. Pressure tendency can forecast short term changes in the weather. Many measurements of air pressure are used within surface weather condition analysis to aid find surface troughs, force per unit area systems and frontal boundaries.
Barometers and pressure altimeters (the most basic and common type of altimeter) are essentially the aforementioned musical instrument, only used for unlike purposes. An altimeter is intended to be used at different levels matching the corresponding atmospheric pressure to the altitude, while a barometer is kept at the same level and measures subtle force per unit area changes caused by weather and elements of weather. The boilerplate atmospheric pressure on the earth's surface varies betwixt 940 and 1040 hPa (mbar). The average atmospheric pressure at sea level is 1013 hPa (mbar).
Etymology
The word "barometer" is derived from the Ancient Greek: βάρος, romanized: báros meaning "weight", and Ancient Greek: μέτρον, romanized: métron significant "measure".
History
Although Evangelista Torricelli is universally credited with inventing the barometer in 1643,[ane] [2] historical documentation as well suggests Gasparo Berti, an Italian mathematician and astronomer, unintentionally built a water barometer onetime between 1640 and 1643.[1] [3] French scientist and philosopher René Descartes described the pattern of an experiment to determine atmospheric pressure as early equally 1631, simply in that location is no prove that he built a working barometer at that fourth dimension.[1]
On 27 July 1630, Giovanni Battista Baliani wrote a letter to Galileo Galilei explaining an experiment he had made in which a siphon, led over a hill most twenty-one meters loftier, failed to work. Galileo responded with an explanation of the miracle: he proposed that information technology was the power of a vacuum that held the h2o up, and at a certain peak the corporeality of water simply became too much and the force could non hold any more, like a cord that tin can support only and so much weight.[4] [5] This was a restatement of the theory of horror vacui ("nature abhors a vacuum"), which dates to Aristotle, and which Galileo restated as resistenza del vacuo.
Galileo's ideas reached Rome in Dec 1638 in his Discorsi. Raffaele Magiotti and Gasparo Berti were excited by these ideas, and decided to seek a better way to endeavour to produce a vacuum other than with a siphon. Magiotti devised such an experiment, and sometime between 1639 and 1641, Berti (with Magiotti, Athanasius Kircher and Niccolò Zucchi nowadays) carried it out.[five]
Four accounts of Berti's experiment exist, just a uncomplicated model of his experiment consisted of filling with h2o a long tube that had both ends plugged, then standing the tube in a bowl already full of water. The lesser end of the tube was opened, and water that had been inside of information technology poured out into the basin. All the same, just part of the water in the tube flowed out, and the level of the water within the tube stayed at an exact level, which happened to exist x.3 m (34 ft),[6] the same height Baliani and Galileo had observed that was express by the siphon. What was well-nigh important virtually this experiment was that the lowering water had left a space in a higher place information technology in the tube which had no intermediate contact with air to fill it upwardly. This seemed to suggest the possibility of a vacuum existing in the space higher up the water.[5]
Torricelli, a friend and student of Galileo, interpreted the results of the experiments in a novel way. He proposed that the weight of the atmosphere, non an alluring force of the vacuum, held the h2o in the tube. In a letter to Michelangelo Ricci in 1644 concerning the experiments, he wrote:
Many have said that a vacuum does non exist, others that information technology does exist in spite of the repugnance of nature and with difficulty; I know of no one who has said that it exists without difficulty and without a resistance from nature. I argued thus: If there can be found a manifest cause from which the resistance tin be derived which is felt if we endeavor to make a vacuum, it seems to me foolish to endeavor to attribute to vacuum those operations which follow patently from some other crusade; and and then past making some very piece of cake calculations, I found that the cause assigned by me (that is, the weight of the temper) ought by itself alone to offer a greater resistance than information technology does when we try to produce a vacuum.[7]
It was traditionally thought (particularly past the Aristotelians) that the air did not have weight: that is, that the kilometers of air above the surface did not exert whatever weight on the bodies below it. Even Galileo had accepted the weightlessness of air every bit a elementary truth. Torricelli questioned that assumption, and instead proposed that air had weight and that it was the latter (non the alluring force of the vacuum) which held (or rather, pushed) upward the column of water. He idea that the level the water stayed at (c. 10.iii thou) was reflective of the strength of the air'south weight pushing on it (specifically, pushing on the water in the basin and thus limiting how much h2o can fall from the tube into information technology). In other words, he viewed the barometer as a balance, an instrument for measurement (as opposed to merely being an instrument to create a vacuum), and considering he was the first to view it this manner, he is traditionally considered the inventor of the barometer (in the sense in which we now employ the term).[5]
Because of rumors circulating in Torricelli'southward gossipy Italian neighbourhood, which included that he was engaged in some form of sorcery or witchcraft, Torricelli realized he had to keep his experiment surreptitious to avoid the risk of being arrested. He needed to employ a liquid that was heavier than water, and from his previous clan and suggestions by Galileo, he deduced that by using mercury, a shorter tube could be used. With mercury, which is virtually fourteen times denser than water, a tube only 80 cm was now needed, not 10.5 m.[8]
In 1646, Blaise Pascal along with Pierre Petit, had repeated and perfected Torricelli's experiment after hearing about information technology from Marin Mersenne, who himself had been shown the experiment by Torricelli toward the end of 1644. Pascal further devised an experiment to examination the Aristotelian proposition that it was vapours from the liquid that filled the space in a barometer. His experiment compared water with wine, and since the latter was considered more than "spiritous", the Aristotelians expected the vino to stand lower (since more vapours would mean more pushing down on the liquid column). Pascal performed the experiment publicly, inviting the Aristotelians to predict the outcome beforehand. The Aristotelians predicted the wine would stand lower. It did non.[5]
However, Pascal went even farther to test the mechanical theory. If, as suspected by mechanical philosophers like Torricelli and Pascal, air had weight, the pressure would exist less at higher altitudes. Therefore, Pascal wrote to his blood brother-in-law, Florin Perier, who lived almost a mountain called the Puy de Dôme, asking him to perform a crucial experiment. Perier was to take a barometer up the Puy de Dôme and make measurements along the way of the height of the column of mercury. He was so to compare it to measurements taken at the foot of the mountain to see if those measurements taken higher up were in fact smaller. In September 1648, Perier carefully and meticulously carried out the experiment, and found that Pascal'due south predictions had been correct. The mercury barometer stood lower the higher one went.[5]
Types
Water barometers

The concept that decreasing atmospheric pressure predicts stormy atmospheric condition, postulated by Lucien Vidi, provides the theoretical basis for a weather prediction device called a "weather drinking glass" or a "Goethe barometer" (named for Johann Wolfgang von Goethe, the renowned High german writer and polymath who developed a simple but constructive conditions ball barometer using the principles developed by Torricelli). The French name, le baromètre Liègeois, is used past some English language speakers.[nine] This name reflects the origins of many early weather condition glasses – the drinking glass blowers of Liège, Kingdom of belgium.[ix] [10]
The weather ball barometer consists of a glass container with a sealed body, half filled with h2o. A narrow spout connects to the trunk below the h2o level and rises above the water level. The narrow spout is open to the temper. When the air pressure level is lower than information technology was at the time the body was sealed, the water level in the spout will ascension higher up the water level in the body; when the air force per unit area is higher, the water level in the spout will drib below the water level in the body. A variation of this blazon of barometer can be hands made at home.[11]
Mercury barometers
A mercury barometer is an instrument used to measure atmospheric pressure in a certain location and has a vertical glass tube closed at the superlative sitting in an open mercury-filled bowl at the bottom. Mercury in the tube adjusts until the weight of it balances the atmospheric forcefulness exerted on the reservoir. High atmospheric pressure places more force on the reservoir, forcing mercury higher in the column. Low pressure allows the mercury to drop to a lower level in the column past lowering the strength placed on the reservoir. Since higher temperature levels effectually the instrument volition reduce the density of the mercury, the scale for reading the height of the mercury is adapted to compensate for this effect. The tube has to be at to the lowest degree as long equally the amount dipping in the mercury + caput space + the maximum length of the column.
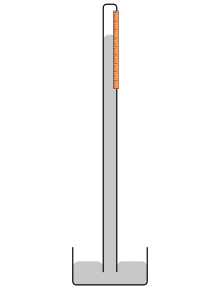
Schematic drawing of a simple mercury barometer with vertical mercury column and reservoir at base of operations
Torricelli documented that the height of the mercury in a barometer changed slightly each twenty-four hours and concluded that this was due to the changing pressure in the atmosphere.[one] He wrote: "We alive submerged at the bottom of an body of water of elementary air, which is known past incontestable experiments to have weight".[12] Inspired by Torricelli, Otto von Guericke on 5 December 1660 establish that air pressure was unusually depression and predicted a storm, which occurred the next day.[13]

The mercury barometer'due south blueprint gives rise to the expression of atmospheric pressure level in inches or millimeters of mercury (mmHg). A torr was originally defined as 1 mmHg. The pressure is quoted as the level of the mercury'southward superlative in the vertical column. Typically, atmospheric pressure level is measured betwixt 26.5 inches (670 mm) and 31.5 inches (800 mm) of Hg. One atmosphere (i atm) is equivalent to 29.92 inches (760 mm) of mercury.

Reservoir of a Fortin barometer
Design changes to brand the instrument more sensitive, simpler to read, and easier to send resulted in variations such every bit the basin, siphon, wheel, cistern, Fortin, multiple folded, stereometric, and balance barometers.
In 2007, a European Union directive was enacted to restrict the employ of mercury in new measuring instruments intended for the general public, effectively ending the production of new mercury barometers in Europe. The repair and trade of antiques (produced before belatedly 1957) remained unrestricted.[fourteen] [xv]
Fitzroy barometer
Fitzroy barometers combine the standard mercury barometer with a thermometer, also equally a guide of how to interpret force per unit area changes.

Sympiesometer inscribed at bottom Improved sympiesometer and at superlative A R Easton, 53 Marischal Street, Aberdeen. Owned past descendants of the Aberdeen shipbuilding Hall family.
Fortin barometer
Fortin barometers use a variable deportation mercury cistern, usually synthetic with a thumbscrew pressing on a leather diaphragm bottom (Five in the diagram). This compensates for displacement of mercury in the column with varying pressure. To apply a Fortin barometer, the level of mercury is set to zero by using the thumbscrew to brand an ivory pointer (O in the diagram) only touch the surface of the mercury. The pressure is then read on the column past adjusting the vernier calibration so that the mercury merely touches the sightline at Z. Some models as well utilise a valve for closing the cistern, enabling the mercury column to exist forced to the height of the cavalcade for ship. This prevents water-hammer damage to the column in transit.
Sympiesometer
A sympiesometer is a compact and lightweight barometer that was widely used on ships in the early 19th century. The sensitivity of this barometer was also used to measure distance.[16]
Sympiesometers have two parts. One is a traditional mercury thermometer that is needed to calculate the expansion or contraction of the fluid in the barometer. The other is the barometer, consisting of a J-shaped tube open up at the lower end and airtight at the peak, with modest reservoirs at both ends of the tube.
Wheel barometers
A wheel barometer uses a "J" tube sealed at the top of the longer limb. The shorter limb is open up to the atmosphere and floating on top of the mercury there is a pocket-size drinking glass float. A fine silken thread is attached to the bladder which passes upwardly over a cycle and then back down to a counterweight (usually protected in another tube). The cycle turns the bespeak on the front of the barometer. As atmospheric pressure increases mercury moves from the brusk to the long limb, the float falls and the pointer moves. When pressure increases the mercury moves dorsum, lifting the float and turning the dial the other fashion.[17]
Around 1810 the cycle barometer, which could exist read from a groovy distance, became the first practical and commercial instrument favoured past farmers and the educated classes in the United kingdom. The face of the barometer was circular with a uncomplicated dial pointing to an hands readable calibration: "Rain - Change - Dry" with the "Change" at the top center of the punch. After models added a barometric scale with effectively graduations "Stormy (28 inches of mercury), Much Rain (28.5), Pelting (29), Change (29.5), Fair (30), Set up fair (thirty.v), very dry out(31)".
Natalo Aiano is recognised equally one of the finest makers of wheel barometers, an early pioneer in a wave of artisanal Italian musical instrument and barometer makers that were encouraged to immigrate to the UK. He listed every bit working in Holborn, London c.1785-1805.[18] From 1770 onwards a large number of Italians came to England because they were accomplished glass blowers or instrument makers. By 1840 it was fair to say that the Italians dominated the industry in England.[19]
Vacuum pump oil barometer
Using vacuum pump oil as the working fluid in a barometer has led to the creation of the new "World's Tallest Barometer" in February 2013. The barometer at Portland State Academy (PSU) uses doubly distilled vacuum pump oil and has a nominal height of about 12.4 g for the oil column height; expected excursions are in the range of ±0.4 m over the form of a yr. Vacuum pump oil has very low vapour pressure level and it is bachelor in a range of densities; the lowest density vacuum oil was called for the PSU barometer to maximize the oil column height.[20]
Aneroid barometers

An aneroid barometer is an instrument used for measuring air pressure as a method that does not involve liquid. Invented in 1844 by French scientist Lucien Vidi,[21] the aneroid barometer uses a modest, flexible metal box chosen an aneroid cell (sheathing), which is made from an alloy of glucinium and copper. The evacuated capsule (or usually several capsules, stacked to add upwards their movements) is prevented from collapsing by a strong spring. Minor changes in external air pressure cause the cell to expand or contract. This expansion and contraction drives mechanical levers such that the tiny movements of the sheathing are amplified and displayed on the confront of the aneroid barometer. Many models include a manually ready needle which is used to mark the current measurement then a change tin can be seen. This type of barometer is common in homes and in recreational boats. It is likewise used in meteorology, mostly in barographs and every bit a force per unit area instrument in radiosondes.
Barographs
A barograph is a recording aneroid barometer where the changes in atmospheric pressure level are recorded on a paper chart.
The principle of the barograph is same as that of the aneroid barometer. Whereas the barometer displays the pressure on a punch, the barograph uses the small movements of the box to transmit by a system of levers to a recording arm that has at its extreme end either a scribe or a pen. A scribe records on smoked foil while a pen records on newspaper using ink, held in a beak. The recording material is mounted on a cylindrical drum which is rotated slowly by a clock. Commonly, the pulsate makes ane revolution per twenty-four hours, per calendar week, or per calendar month and the rotation rate tin often exist selected past the user.
MEMS barometers

Microelectromechanical systems (or MEMS) barometers are extremely small devices between one and 100 micrometres in size (0.001 to 0.1 mm). They are created via photolithography or photochemical machining. Typical applications include miniaturized atmospheric condition stations, electronic barometers and altimeters.[22]
A barometer tin can also exist found in smartphones such every bit the Samsung Galaxy Nexus,[23] Samsung Galaxy S3-S6, Motorola Xoom, Apple iPhone 6 and newer iPhones, and Timex Expedition WS4 smartwatch, based on MEMS and piezoresistive pressure-sensing technologies.[24] [25] Inclusion of barometers on smartphones was originally intended to provide a faster GPS lock.[26] However, third political party researchers were unable to confirm additional GPS accuracy or lock speed due to barometric readings. The researchers suggest that the inclusion of barometers in smartphones may provide a solution for determining a user's elevation, simply also suggest that several pitfalls must first be overcome.[27]
More unusual barometers

Timex Expedition WS4 in Barometric chart mode with weather forecast function
At that place are many other more unusual types of barometer. From variations on the storm barometer, such every bit the Collins Patent Tabular array Barometer, to more traditional-looking designs such every bit Hooke's Otheometer and the Ross Sympiesometer. Some, such equally the Shark Oil barometer,[28] work only in a certain temperature range, accomplished in warmer climates.
Applications

Digital graphing barometer.

Analogue recording barograph using 5 stacked aneroid barometer cells.
Barometric pressure and the pressure tendency (the change of pressure over fourth dimension) have been used in weather forecasting since the late 19th century.[29] When used in combination with wind observations, reasonably accurate short-term forecasts can be made.[30] Simultaneous barometric readings from beyond a network of weather condition stations allow maps of air pressure to exist produced, which were the start course of the modern atmospheric condition map when created in the 19th century. Isobars, lines of equal pressure, when drawn on such a map, requite a contour map showing areas of high and low pressure level.[31] Localized high atmospheric pressure level acts as a bulwark to approaching weather condition systems, diverting their grade. Atmospheric lift caused past low-level air current convergence into the surface brings clouds and sometimes precipitation.[32] The larger the change in pressure level, especially if more than than iii.5 hPa (0.1 inHg), the greater the change in conditions that can be expected. If the pressure level drop is rapid, a low pressure level arrangement is approaching, and there is a greater chance of rain. Rapid pressure rises, such as in the wake of a cold front end, are associated with improving weather conditions, such every bit clearing skies.[33]
With falling air pressure, gases trapped inside the coal in deep mines can escape more than freely. Thus low pressure increases the take a chance of firedamp accumulating. Collieries therefore go along runway of the pressure. In the case of the Trimdon Grange colliery disaster of 1882 the mines inspector drew attending to the records and in the report stated "the weather of atmosphere and temperature may be taken to take reached a unsafe point".[34]
Aneroid barometers are used in scuba diving. A submersible pressure guess is used to keep runway of the contents of the diver'southward air tank. Some other gauge is used to measure out the hydrostatic pressure, commonly expressed equally a depth of sea water. Either or both gauges may be replaced with electronic variants or a dive reckoner.[35]
Compensations
Temperature
The density of mercury will alter with increase or decrease in temperature, then a reading must be adjusted for the temperature of the instrument. For this purpose a mercury thermometer is usually mounted on the instrument. Temperature compensation of an aneroid barometer is achieved by including a bi-metal element in the mechanical linkages. Aneroid barometers sold for domestic use typically have no compensation under the supposition that they volition be used within a controlled room temperature range.
Distance

A digital barometer with altimeter setting (for correction) displayed
As the air pressure decreases at altitudes to a higher place bounding main level (and increases below sea level) the uncorrected reading of the barometer volition depend on its location. The reading is and then adjusted to an equivalent sea-level force per unit area for purposes of reporting. For case, if a barometer located at ocean level and nether off-white weather atmospheric condition is moved to an altitude of 1,000 anxiety (305 m), well-nigh one inch of mercury (~35 hPa) must be added on to the reading. The barometer readings at the ii locations should exist the same if there are negligible changes in time, horizontal distance, and temperature. If this were non done, there would be a simulated indication of an approaching storm at the higher acme.
Aneroid barometers have a mechanical adjustment that allows the equivalent sea level pressure level to exist read straight and without further adjustment if the instrument is not moved to a dissimilar altitude. Setting an aneroid barometer is like to resetting an analog clock that is non at the correct time. Its dial is rotated so that the current atmospheric pressure from a known accurate and nearby barometer (such as the local weather station) is displayed. No calculation is needed, as the source barometer reading has already been converted to equivalent body of water-level pressure, and this is transferred to the barometer being gear up—regardless of its altitude. Though somewhat rare, a few aneroid barometers intended for monitoring the weather condition are calibrated to manually adjust for altitude. In this example, knowing either the altitude or the current atmospheric pressure would be sufficient for future accurate readings.
The tabular array below shows examples for iii locations in the urban center of San Francisco, California. Note the corrected barometer readings are identical, and based on equivalent bounding main-level pressure. (Assume a temperature of 15 °C.)
| Location | Altitude (anxiety) | Uncorrected Patm (inches Hg) | Corrected Patm (inches Hg) | Altitude (metres) | Uncorrected Patm (hPa) | Corrected Patm (hPa) | |
|---|---|---|---|---|---|---|---|
| City Marina | Sea Level (0) | 29.92 | 29.92 | 0 m | 1013 hPa | 1013 hPa | |
| Nob Colina | 348 | 29.55 | 29.92 | 106 m | 1001 hPa | 1013 hPa | |
| Mt. Davidson | 928 | 28.94 | 29.92 | 283 thousand | 980 hPa | 1013 hPa |
In 1787, during a scientific expedition on Mont Blanc, De Saussure undertook research and executed concrete experiments on the humid point of water at different heights. He calculated the pinnacle at each of his experiments by measuring how long it took an booze burner to boil an amount of water, and by these ways he determined the height of the mountain to be 4775 metres. (This later turned out to be 32 metres less than the actual height of 4807 metres). For these experiments De Saussure brought specific scientific equipment, such equally a barometer and thermometer. His calculated humid temperature of water at the elevation of the mountain was fairly accurate, only off past 0.1 kelvin.[36]
Based on his findings, the altimeter could be adult equally a specific awarding of the barometer. In the mid-19th century, this method was used past explorers.[37]
Equation
When atmospheric pressure level is measured by a barometer, the pressure level is also referred to as the "barometric pressure". Assume a barometer with a cantankerous-exclusive area A, a peak h, filled with mercury from the bottom at Indicate B to the pinnacle at Point C. The force per unit area at the bottom of the barometer, Signal B, is equal to the atmospheric pressure. The pressure at the very top, Betoken C, can be taken as zip because in that location is only mercury vapour above this betoken and its pressure is very low relative to the atmospheric force per unit area. Therefore, one can find the atmospheric pressure using the barometer and this equation:[38] [ clarification needed ]
- Patm = ρgh
where ρ is the density of mercury, chiliad is the gravitational acceleration, and h is the height of the mercury column above the complimentary surface area. The physical dimensions (length of tube and cross-sectional surface area of the tube) of the barometer itself have no issue on the height of the fluid column in the tube.
In thermodynamic calculations, a unremarkably used force per unit area unit of measurement is the "standard atmosphere". This is the pressure level resulting from a column of mercury of 760 mm in height at 0 °C. For the density of mercury, use ρHg = xiii,595 kg/10003 and for gravitational dispatch use yard = 9.807 m/due south2.
If water were used (instead of mercury) to run into the standard atmospheric pressure, a water column of roughly ten.3 m (33.8 ft) would be needed.
Standard atmospheric pressure as a role of elevation:
Annotation: 1 torr = 133.3 Pa = 0.03937 inHg
| Patm / kPa | Altitude | Patm / inHg | Altitude | |
|---|---|---|---|---|
| 101.325 | Sea Level (0m) | 29.92 | Sea Level (0 ft) | |
| 97.71 | 305 thousand | 28.86 | one,000 ft | |
| 94.21 | 610 m | 27.82 | 2,000 ft | |
| 89.88 | ane,000 thousand | 26.55 | iii,281 ft | |
| 84.31 | one,524 one thousand | 24.xc | 5,000 ft | |
| 79.l | ii,000 g | 23.48 | 6,562 ft | |
| 69.68 | three,048 thou | 20.58 | ten,000 ft | |
| 54.05 | 5,000 chiliad | 15.96 | 16,404 ft | |
| 46.56 | 6,096 m | 13.75 | 20,000 ft | |
| 37.65 | 7,620 m | 11.12 | 25,000 ft | |
| 32.77 | 8,848 g* | 9.68 | 29,029 ft* | |
| 26.44 | 10,000 m | seven.81 | 32,808 ft | |
| 11.65 | xv,240 m | iii.44 | 50,000 ft | |
| 5.53 | twenty,000 one thousand | 1.63 | 65,617 ft |
- Elevation of Mount Everest, the highest indicate on earth
Patents

Table of Pneumaticks, 1728 Cyclopaedia
- United states 2194624, G. A. Titterington, Jr, "Diaphragm pressure gauge having temperature compensating means", issued 1940-03-26, assigned to Bendix Aviat Corp
- U.S. Patent 2,472,735 : C. J. Ulrich : "Barometric instrument"
- U.Southward. Patent 2,691,305 : H. J. Frank : "Barometric altimeter"
- U.Due south. Patent 3,273,398 : D. C. Due west. T. Sharp : "Aneroid barometer"
- U.S. Patent 3,397,578 : H. A. Klumb : "Move amplifying mechanism for pressure level responsive instrument move"
- U.S. Patent 3,643,510 : F. Lissau : "Fluid displacement pressure level gauges"
- U.Southward. Patent four,106,342 : O. Due south. Sormunen : "Pressure level measuring instrument"
- U.S. Patent 4,238,958 : H. Dostmann : "Barometer"
- U.Due south. Patent 4,327,583 : T. Fijimoto : "Weather forecasting device"
See also
- Altimeter
- Anemoscope
- Automatic airdrome conditions station
- Barograph
- Barometer question
- Bert Bolle Barometer
- Microbarometer
- Storm drinking glass
- Surface weather analysis
- Tempest prognosticator
- Units of pressure
- Pressure level sensor
- Atmospheric condition forecasting
- Zambretti Forecaster
References
- ^ a b c d "The Invention of the Barometer". Islandnet.com. Retrieved 2010-02-04 .
- ^ "History of the Barometer". Barometerfair.com. Archived from the original on 2009-09-25. Retrieved 2010-02-04 .
- ^ Drake, Stillman (1970). "Berti, Gasparo". Dictionary of Scientific Biography. Vol. two. New York: Charles Scribner'south Sons. pp. 83–84. ISBN978-0-684-10114-9.
- ^ Shea, William R. (2003). Designing Experiments & Games of Take a chance: The Unconventional Scientific discipline of Blaise Pascal. Science History Publications. pp. 21–. ISBN978-0-88135-376-ane . Retrieved 10 October 2012.
- ^ a b c d e f "History of the Barometer". Strange-loops.com. 2002-01-21. Archived from the original on 6 January 2010. Retrieved 2010-02-04 .
- ^ Gillispie, Charles Coulston (1960). The Edge of Objectivity: An Essay in the History of Scientific Ideas . Princeton University Press. pp. 99–100. ISBN0-691-02350-6.
- ^ "Torricelli's letter to Michelangelo Ricci". Web.lemoyne.edu. Retrieved 2010-02-04 .
- ^ "Brief History of the Barometer". Barometer.ws. Archived from the original on 14 January 2010. Retrieved 2010-02-04 .
- ^ a b Gerard 50'E. Turner, Nineteenth Century Scientific Instruments, Sotheby Publications, 1983, p 236, ISBN 0-85667-170-3
- ^ Claus Zittle, Philosophies of Engineering science: Francis Salary and His Contemporaries, BRILL 2008, pp 115, 116 ISBN xc-04-17050-2
- ^ Jet Stream. Learning Lesson: Measure the Pressure – The "Moisture" Barometer. Retrieved on 2019-01-21.
- ^ Strangeways, Ian. Measuring the Natural Environment. Cambridge University Press, 2000, p. 92.
- ^ Ley, Willy (June 1966). "The Re-Designed Solar Organisation". For Your Information. Milky way Science Fiction. pp. 94–106.
- ^ Jones H. (10 July 2007). "EU bans mercury in barometers, thermometers". Reuters . Retrieved 12 September 2017.
- ^ "Ban on auction of mercury measuring instruments - MEPs agree two year exemption for barometers". European Parliament. 10 July 2007. Retrieved 2021-05-11 .
- ^ Stanton, William (1975). The Neat U.s. Exploring Trek . Berkeley: University of California Press. pp. 126. ISBN0520025571.
- ^ Hood, Jean (five December 2017). "Barometers : History, working and styles". Retrieved 21 June 2020.
- ^ "Natalo Aiano". Almost u.s. folio. C. Aiano & Sons Ltd. 22 May 2017.
- ^ Nicholas, Goodison (1977). English barometers 1680-1860 : a history of domestic barometers and their makers and retailers (Rev. and enl. ed.). Antique Collectors' Lodge. ISBN978-0902028524.
- ^ Tomlinson, Stuart (February ten, 2013) Big barometer at Portland Land University could be the tallest in the earth. oregonlive.com
- ^ Figuier, Louis; Gautier, Émile (1867). 50'Année scientifique et industrielle. L. Hachette et cie. pp. 485–486.
- ^ "MEMS Barometric Pressure Sensor". Sensors & Transducers E-Digest. 92 (4). 2008. Retrieved 13 June 2014.
- ^ This Is the Samsung Galaxy Nexus, Google's New Official Android Phone. Gizmodo.com (2011-10-xviii). Retrieved on 2011-11-15.
- ^ Molen, Brad (2011-ten-20). "Backside the glass: a detailed tour inside the Samsung Galaxy Nexus". Engadget. Engadget. Archived from the original on 2014-12-05. Retrieved 2015-06-23 .
Barometric pressure sensor: BOSCH BMP180
- ^ "BMP180: Digital, barometric force per unit area sensor" (PDF). Bosch. Archived from the original (PDF) on 2015-06-23. Retrieved 2015-06-23 .
- ^ Galaxy Nexus barometer explained, Sam Champion not out of a job. Engadget (2011-10-20). Retrieved on 2011-12-03.
- ^ Muralidharan, Kartik; Khan, Azeem Javed; Misra, Archan; Balan, Rajesh Krishna; Agarwal, Sharad (2014-02-26). "Barometric Phone Sensors – More Hype Than Hope!". ACM HotMobile: two. Retrieved 2015-06-23 .
- ^ Shark Oil Barometer Archived July 20, 2011, at the Wayback Machine Barometer World.
- ^ Understanding air pressure. USA Today.
- ^ Using winds and a barometer to make forecasts. USA Today (17 May 2005).
- ^ Hopkins, Edward J. (1996-06-10). "Surface Weather Analysis Chart". University of Wisconsin. Archived from the original on 28 April 2007. Retrieved 2007-05-10 .
- ^ Pearce, Robert Penrose (2002). Meteorology at the Millennium. Academic Press. p. 66. ISBN978-0-12-548035-2 . Retrieved 2009-01-02 .
- ^ Applying The Barometer To Conditions Watching. Weather Md.
- ^ Report on the Explosion which occurred at the Trimdon Grange Colliery on the 16th February 1882 , retrieved 23 July 2015
- ^ The Encyclopedia of Recreational Diving . Santa Ana, CA, United states of america: Professional person Association of Diving Instructors. 1990. pp. 3–96–3–99. ISBN978-i-878663-02-3.
- ^ "Kelvin scale in depth". Retrieved 12 February 2020.
- ^ Berberan-Santos, Thousand. N.; Bodunov, E. N.; Pogliani, L. (1997). "On the barometric formula". American Journal of Physics. 65 (5): 404–412. Bibcode:1997AmJPh..65..404B. doi:10.1119/1.18555.
- ^ Cengal, Yunus A. and Boles, Michael A. (2014) Thermodynamics: An Engineering Arroyo. McGraw-Hill Didactics. ISBN 978-0073398174
Further reading
- . Encyclopædia Britannica. Vol. 3 (11th ed.). 1911.
- Burch, David F. The Barometer Handbook: A Modern Wait at Barometers and Applications of Barometric Pressure. Seattle: Starpath Publications (2009), ISBN 978-0-914025-12-2.
- Middleton, West. Eastward. Knowles. (1964). The History of the Barometer. Baltimore: Johns Hopkins Printing. New edition (2002), ISBN 0-8018-7154-9.
External links
| | Wikimedia Commons has media related to Barometers. |
-
 Works related to Observations upon the Marine Barometer ... at Wikisource
Works related to Observations upon the Marine Barometer ... at Wikisource
Source: https://en.wikipedia.org/wiki/Barometer
Posted by: fosdickgagainfoute.blogspot.com

0 Response to "A Weather Instrument Used To Measure Changes In Air Pressure?"
Post a Comment