How To Change A 28 Spline To A 31
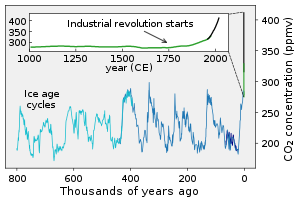
COii concentrations over the terminal 800,000 years
Carbon dioxide (COii) is an important trace gas in Globe'southward atmosphere. It is an integral office of the carbon cycle, a biogeochemical cycle in which carbon is exchanged between the Earth'due south oceans, soil, rocks and the biosphere. Plants and other photoautotrophs use solar free energy to produce carbohydrate from atmospheric carbon dioxide and water past photosynthesis. Almost all other organisms depend on carbohydrate derived from photosynthesis as their primary source of free energy and carbon compounds. CO2 absorbs and emits infrared radiation at wavelengths of 4.26 μm (2347 cm−1) (disproportionate stretching vibrational manner) and xiv.99 μm (667 cm−1) (bending vibrational mode) and consequently is a greenhouse gas that plays a pregnant role in influencing Earth's surface temperature through the greenhouse issue.[1]
Concentrations of CO2 in the atmosphere were as high as four,000 parts per 1000000 (ppm, on a molar footing) during the Cambrian menstruation about 500 one thousand thousand years ago to every bit depression as 180 ppm during the Quaternary glaciation of the last two meg years.[2] Reconstructed temperature records for the last 420 meg years indicate that atmospheric CO2 concentrations peaked at ~2000 ppm during the Devonian (~400 Myrs ago) period, and once more in the Triassic (220–200 Myrs ago) period. Global annual mean CO2 concentration has increased by 50% since the start of the Industrial Revolution, from 280 ppm during the x,000 years upward to the mid-18th century[two] to 420 ppm equally of April 2021.[iii] The present concentration is the highest for 14 million years.[4] The increase has been attributed to human activity, particularly deforestation and the burning of fossil fuels.[v] This increase of CO2 and other long-lived greenhouse gases in Globe'due south temper has produced the current episode of global warming. Between 30% and forty% of the COii released past humans into the atmosphere dissolves into the oceans,[6] [seven] wherein information technology forms carbonic acid and furnishings changes in the oceanic pH balance.
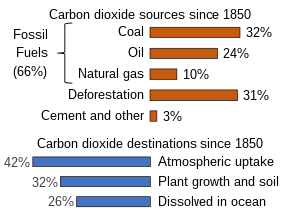
Between 1850 and 2019 the Global Carbon Projection estimates that about two/3rds of excess carbon dioxide emissions have been caused by burning fossil fuels, and a lilliputian less than one-half of that has stayed in the atmosphere.
Electric current concentration [edit]

A model of the behavior of carbon in the atmosphere from one September 2014 to 31 August 2015. The height of Earth'due south atmosphere and topography have been vertically exaggerated and appear approximately 40 times higher than normal to show the complication of the atmospheric flow.

Carbon dioxide concentrations have shown several cycles of variation from about 180 parts per 1000000 during the deep glaciations of the Holocene and Pleistocene to 280 parts per million during the interglacial periods. Post-obit the outset of the Industrial Revolution, atmospheric COtwo concentration increased to over 400 parts per meg and continues to increment, causing the phenomenon of global warming.[viii] As of April 2019[update], the average monthly level of COii in Earth's atmosphere exceeded 413 parts per million.[9] The daily average concentration of atmospheric CO2 at Mauna Loa Observatory showtime exceeded 400 ppm on 10 May 2013[10] [eleven] although this concentration had already been reached in the Arctic in June 2012.[12] Each part per million by volume of CO2 in the atmosphere represents approximately two.13 gigatonnes of carbon, or vii.82 gigatonnes of COtwo.[13] As of 2018, CO2 constitutes well-nigh 0.041% by book of the atmosphere, (equal to 410 ppm)[xiv] [xv] [sixteen] [17] [eighteen] which corresponds to approximately 3210 gigatonnes of CO2, containing approximately 875 gigatonnes of carbon. The global hateful CO2 concentration is currently rising at a charge per unit of approximately 2 ppm/year and accelerating.[14] [19] The current growth rate at Mauna Loa is two.l ± 0.26 ppm/year (mean ± 2 std dev).[20] Equally seen in the graph to the right, there is an annual fluctuation – the level drops by nigh six or seven ppm (about 50 Gt) from May to September during the Northern Hemisphere'southward growing season, and then goes upward by nigh 8 or 9 ppm. The Northern Hemisphere dominates the almanac bike of CO2 concentration considering information technology has much greater state area and institute biomass than the Southern Hemisphere. Concentrations reach a tiptop in May as the Northern Hemisphere bound greenup begins, and refuse to a minimum in October, near the cease of the growing season.[xx] [21]
Since global warming is attributed to increasing atmospheric concentrations of greenhouse gases such equally CO2 and methyl hydride, scientists closely monitor atmospheric COtwo concentrations and their impact on the present-twenty-four hours biosphere. The National Geographic wrote that the concentration of carbon dioxide in the atmosphere is this high "for the showtime time in 55 years of measurement—and probably more than 3 million years of Earth history."[22] The current concentration may be the highest in the last twenty million years.[23]
By concentration [edit]

Concentration of atmospheric CO2 over the last twoscore,000 years, from the Last Glacial Maximum to the present day. The current rate of increase is much higher than at any indicate during the last deglaciation.
Carbon dioxide concentrations have varied widely over the Earth'south 4.54 billion yr history. It is believed to have been nowadays in Globe's first atmosphere, shortly later on World's germination. The 2nd atmosphere, consisting largely of nitrogen and CO
2 was produced by outgassing from volcanism, supplemented past gases produced during the late heavy battery of Globe by huge asteroids.[24] A major office of carbon dioxide emissions were presently dissolved in water and incorporated in carbonate sediments.
The production of gratuitous oxygen past cyanobacterial photosynthesis somewhen led to the oxygen catastrophe that ended Earth's second atmosphere and brought about the Earth'southward third atmosphere (the modern atmosphere) 2.iv billion years before the present. Carbon dioxide concentrations dropped from 4,000 parts per million during the Cambrian period most 500 million years ago to as depression as 180 parts per million during the 4th glaciation of the last two 1000000 years.[2]
Drivers of ancient-Earth COii concentration [edit]
On long timescales, atmospheric COii concentration is determined by the balance among geochemical processes including organic carbon burial in sediments, silicate rock weathering, and volcanic degassing. The net upshot of slight imbalances in the carbon cycle over tens to hundreds of millions of years has been to reduce atmospheric CO2. On a timescale of billions of years, such downwards tendency appears bound to go on indefinitely as occasional massive historical releases of buried carbon due to volcanism volition become less frequent (as earth curtain cooling and progressive exhaustion of internal radioactive estrus go on further). The rates of these processes are extremely slow; hence they are of no relevance to the atmospheric CO2 concentration over the next hundreds or thousands of years.
In billion-year timescales, it is predicted that plant, and therefore animal, life on land will die off altogether, since by that time about of the remaining carbon in the atmosphere will be sequestered hugger-mugger, and natural releases of CO2 by radioactivity-driven tectonic activity volition have continued to slow down.[25] [ better source needed ] The loss of institute life would also result in the eventual loss of oxygen. Some microbes are capable of photosynthesis at concentrations of CO2 of a few parts per million and so the last life forms would probably disappear finally due to the ascension temperatures and loss of the atmosphere when the sun becomes a blood-red giant some four billion years from now.[26]
Measuring ancient-Earth COii concentration [edit]
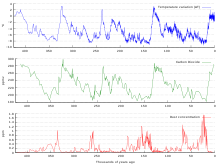
Graph of CO2 (light-green), reconstructed temperature (blue) and dust (red) from the Vostok ice cadre for the past 420,000 years
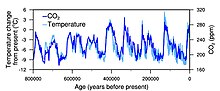
Correspondence between temperature and atmospheric CO2 during the final 800,000 years
The nigh direct method for measuring atmospheric carbon dioxide concentrations for periods before instrumental sampling is to measure bubbling of air (fluid or gas inclusions) trapped in the Antarctic or Greenland ice sheets. The almost widely accepted of such studies come up from a variety of Antarctic cores and indicate that atmospheric COtwo concentrations were about 260–280 ppmv immediately before industrial emissions began and did non vary much from this level during the preceding 10,000 years.[27] The longest ice core tape comes from East Antarctica, where ice has been sampled to an age of 800,000 years.[28] During this time, the atmospheric carbon dioxide concentration has varied betwixt 180 and 210 ppm during ice ages, increasing to 280–300 ppm during warmer interglacials.[29] [xxx] The beginning of human agriculture during the electric current Holocene epoch may have been strongly connected to the atmospheric CO2 increase subsequently the last water ice age concluded, a fertilization event raising institute biomass growth and reducing stomatal conductance requirements for CO2 intake, consequently reducing transpiration water losses and increasing h2o usage efficiency.[31]
Various proxy measurements have been used to endeavor to determine atmospheric carbon dioxide concentrations millions of years in the past. These include boron and carbon isotope ratios in certain types of marine sediments, and the number of stomata observed on fossil plant leaves.[32]
Phytane is a blazon of diterpenoid alkane series. It is a breakup product of chlorophyll and is now used to judge ancient CO2 levels.[33] Phytane gives both a continuous record of CO2 concentrations just it also can overlap a interruption in the CO2 record of over 500 million years.[33]
400 to 600 M yrs ago [edit]
In that location is evidence for high CO2 concentrations betwixt 200 and 150 million years agone of over iii,000 ppm, and between 600 and 400 million years ago of over 6,000 ppm.[23]
5 to threescore M yrs ago [edit]
In more recent times, atmospheric COii concentration continued to autumn after virtually 60 million years ago. Nearly 34 1000000 years ago, the fourth dimension of the Eocene–Oligocene extinction event and when the Antarctic water ice canvas started to accept its current form, CO2 was nearly 760 ppm,[34] and at that place is geochemical evidence that concentrations were less than 300 ppm by about 20 million years ago. Decreasing CO2 concentration, with a tipping point of 600 ppm, was the primary agent forcing Antarctic glaciation.[35] Low CO2 concentrations may have been the stimulus that favored the evolution of C4 plants, which increased greatly in abundance between seven and 5 million years ago.[32]
CO2 possibly college 7,000 - x,000 yrs ago [edit]
Based on an analysis of fossil leaves, Wagner et al.[36] argued that atmospheric CO2 concentrations during the final 7,000–x,000 twelvemonth period were significantly higher than 300 ppm and contained substantial variations that may exist correlated to climate variations. Others have disputed such claims, suggesting they are more likely to reflect calibration problems than bodily changes in CO2.[37] Relevant to this dispute is the observation that Greenland ice cores often report college and more variable CO2 values than similar measurements in Antarctica. However, the groups responsible for such measurements (due east.g. H.J. Smith et al.[38]) believe the variations in Greenland cores result from in situ decomposition of calcium carbonate dust found in the ice. When dust concentrations in Greenland cores are low, as they nearly always are in Antarctic cores, the researchers report good agreement between measurements of Antarctic and Greenland CO2 concentrations.
Atmospheric COtwo and the greenhouse upshot [edit]

A pictogram of the greenhouse consequence
Earth's natural greenhouse effect makes life equally we know it possible and carbon dioxide plays a significant office in providing for the relatively high temperature that the planet enjoys. The greenhouse event is a process by which thermal radiation from a planetary atmosphere warms the planet's surface across the temperature information technology would have in the absence of its atmosphere.[39] [40] [41] Without the greenhouse outcome, the Earth's average surface temperature would exist about −eighteen °C (−0.4 °F)[42] [43] compared to Earth'southward actual average surface temperature of approximately 14 °C (57.2 °F).[44]
Carbon dioxide is believed to have played an of import consequence in regulating Earth's temperature throughout its 4.vii billion year history. Early in the Earth'south life, scientists have found testify of liquid h2o indicating a warm world even though the Sun's output is believed to have merely been seventy% of what information technology is today. It has been suggested by scientists that higher carbon dioxide concentrations in the early on Globe'due south atmosphere might aid explain this faint immature sun paradox. When Globe offset formed, Earth's temper may take independent more greenhouse gases and CO2 concentrations may have been higher, with estimated partial pressure as large as ane,000 kPa (10 bar), because there was no bacterial photosynthesis to reduce the gas to carbon compounds and oxygen. Methane, a very agile greenhouse gas which reacts with oxygen to produce CO2 and water vapor, may have been more prevalent equally well, with a mixing ratio of 10−iv (100 parts per one thousand thousand by book).[45] [46]

Radiative forcing drivers of climate change in year 2011, relative to pre-industrial (1750).
Though h2o is responsible for most (about 36-70%) of the full greenhouse consequence, the role of water vapor as a greenhouse gas depends on temperature. On Earth, carbon dioxide is the near relevant, direct anthropologically influenced greenhouse gas. Carbon dioxide is often mentioned in the context of its increased influence as a greenhouse gas since the pre-industrial (1750) era. In the IPCC Fifth Assessment Report the increase in CO2 was estimated to be responsible for 1.82 West m−2 of the ii.63 Due west one thousand−2 change in radiative forcing on Earth (well-nigh 70%).[47]
The concept of atmospheric COtwo increasing basis temperature was first published by Svante Arrhenius in 1896.[48] The increased radiative forcing due to increased CO2 in the World'southward temper is based on the physical properties of CO2 and the non-saturated absorption windows where CO2 absorbs outgoing long-wave energy. The increased forcing drives farther changes in World's energy residual and, over the longer term, in Earth's climate.[47]
Atmospheric CO2 and the carbon bicycle [edit]
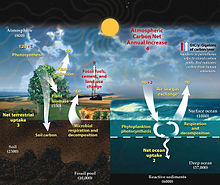
This diagram of the fast carbon cycle shows the movement of carbon betwixt state, temper, and oceans in billions of metric tons of carbon per year. Yellow numbers are natural fluxes, red are human contributions, white are stored carbon.[49]
Atmospheric carbon dioxide plays an integral office in the Earth'southward carbon cycle whereby CO2 is removed from the atmosphere by some natural processes such as photosynthesis and degradation of carbonates, to grade limestones for case, and added back to the atmosphere by other natural processes such every bit respiration and the acid dissolution of carbonate deposits. At that place are two broad carbon cycles on Globe: the fast carbon cycle and the slow carbon cycle. The fast carbon cycle refers to movements of carbon between the environment and living things in the biosphere whereas the ho-hum carbon wheel involves the movement of carbon between the atmosphere, oceans, soil, rocks, and volcanism. Both cycles are intrinsically interconnected and atmospheric CO2 facilitates the linkage.
Natural sources of atmospheric COii include volcanic outgassing, the combustion of organic thing, wildfires and the respiration processes of living aerobic organisms. Man-made sources of COtwo include the burning of fossil fuels for heating, power generation and ship, as well as some industrial processes such as cement making. Information technology is also produced by various microorganisms from fermentation and cellular respiration. Plants, algae and blue-green alga catechumen carbon dioxide to carbohydrates by a process called photosynthesis. They gain the energy needed for this reaction from absorption of sunlight past chlorophyll and other pigments. Oxygen, produced as a by-production of photosynthesis, is released into the temper and subsequently used for respiration by heterotrophic organisms and other plants, forming a cycle with carbon.
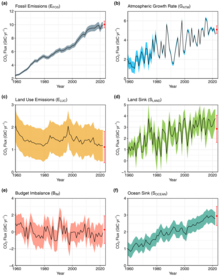
Annual COii flows from anthropogenic sources (left) into Earth's atmosphere, land, and ocean sinks (correct) since twelvemonth 1960. Units in equivalent gigatonnes carbon per year.[50]
Most sources of CO2 emissions are natural, and are counterbalanced to various degrees by similar COii sinks. For case, the decay of organic material in forests, grasslands, and other country vegetation - including forest fires - results in the release of about 436 gigatonnes of COtwo (containing 119 gigatonnes carbon) every year, while COii uptake past new growth on state counteracts these releases, absorbing 451 Gt (123 Gt C).[51] Although much CO2 in the early on atmosphere of the young Earth was produced past volcanic action, modern volcanic activity releases only 130 to 230 megatonnes of CO2 each year.[52] Natural sources are more or less balanced past natural sinks, in the form of chemical and biological processes which remove CO2 from the atmosphere. By contrast, equally of yr 2019 the extraction and called-for of geologic fossil carbon by humans releases over 30 gigatonnes of CO2 (9 billion tonnes carbon) each year.[50] This larger disruption to the natural remainder is responsible for recent growth in the atmospheric CO2 concentration.[16] [53]
Overall, at that place is a large natural flux of atmospheric CO2 into and out of the biosphere, both on country and in the oceans.[54] In the pre-industrial era, each of these fluxes were in residual to such a caste that little cyberspace COii flowed between the land and sea reservoirs of carbon, and little change resulted in the atmospheric concentration. From the human pre-industrial era to 1940, the terrestrial biosphere represented a cyberspace source of atmospheric COii (driven largely by land-use changes), but subsequently switched to a internet sink with growing fossil carbon emissions.[55] In 2012, most 57% of human being-emitted CO2, mostly from the burning of fossil carbon, was taken up by land and body of water sinks.[56] [55]
The ratio of the increment in atmospheric CO2 to emitted CO2 is known as the airborne fraction (Keeling et al., 1995). This ratio varies in the short-term and is typically well-nigh 45% over longer (5-year) periods.[55] Estimated carbon in global terrestrial vegetation increased from approximately 740 gigatonnes in 1910 to 780 gigatonnes in 1990.[57] By 2009, oceanic neutralization had decreased the pH of seawater by 0.11 due to uptake of emitted CO2.[58]
Atmospheric CO2 and photosynthesis [edit]
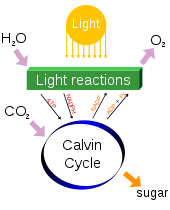
Photosynthesis changes sunlight into chemical energy, splits water to liberate O2, and fixes CO2 into sugar.
Carbon dioxide in the Globe'due south atmosphere is essential to life and to near of the planetary biosphere. Over the grade of Earth'southward geologic history CO2 concentrations accept played a role in biological evolution. The start photosynthetic organisms probably evolved early on in the evolutionary history of life and well-nigh likely used reducing agents such as hydrogen or hydrogen sulfide as sources of electrons, rather than h2o.[59] Cyanobacteria appeared later, and the backlog oxygen they produced contributed to the oxygen catastrophe,[60] which rendered the evolution of complex life possible. In recent geologic times, low CO2 concentrations below 600 parts per million might have been the stimulus that favored the evolution of C4 plants which increased greatly in abundance betwixt seven and five million years ago over plants that use the less efficient C3 metabolic pathway.[32] At current atmospheric pressures photosynthesis shuts downwards when atmospheric CO2 concentrations fall below 150 ppm and 200 ppm although some microbes can extract carbon from the air at much lower concentrations.[61] [62] Today, the average rate of energy capture past photosynthesis globally is approximately 130 terawatts,[63] [64] [65] which is about six times larger than the current power consumption of man civilization.[66] Photosynthetic organisms also catechumen effectually 100–115 billion metric tonnes of carbon into biomass per yr.[67] [68]
Photosynthetic organisms are photoautotrophs, which means that they are able to synthesize food directly from CO2 and water using energy from low-cal. However, non all organisms that utilise light every bit a source of energy carry out photosynthesis, since photoheterotrophs use organic compounds, rather than CO2, equally a source of carbon.[69] In plants, algae and cyanobacteria, photosynthesis releases oxygen. This is called oxygenic photosynthesis. Although there are some differences between oxygenic photosynthesis in plants, algae, and cyanobacteria, the overall process is quite like in these organisms. However, there are some types of leaner that carry out anoxygenic photosynthesis, which consumes CO2 but does not release oxygen.
Carbon dioxide is converted into sugars in a process called carbon fixation. Carbon fixation is an endothermic redox reaction, so photosynthesis needs to supply both the source of energy to bulldoze this process and the electrons needed to convert COtwo into a carbohydrate. This addition of the electrons is a reduction reaction. In full general outline and in consequence, photosynthesis is the reverse of cellular respiration, in which glucose and other compounds are oxidized to produce CO2 and water, and to release exothermic chemical energy to drive the organism's metabolism. However, the two processes take place through a different sequence of chemical reactions and in different cellular compartments.
Most organisms that utilize photosynthesis to produce oxygen use visible light to do then, although at to the lowest degree three use shortwave infrared or, more than specifically, far-ruby radiations.[70]
Effects of increased CO2 on plants and crops [edit]
A 1993 review of scientific greenhouse studies plant that a doubling of CO2 concentration would stimulate the growth of 156 different found species past an boilerplate of 37%. Response varied significantly by species, with some showing much greater gains and a few showing a loss. For example, a 1979 greenhouse study found that with doubled CO2 concentration the dry weight of forty-twenty-four hour period-old cotton plants doubled, but the dry weight of xxx-day-old maize plants increased past only 20%.[71] [72]
In improver to greenhouse studies, field and satellite measurements attempt to understand the effect of increased COii in more natural environments. In free-air carbon dioxide enrichment (Confront) experiments plants are grown in field plots and the CO2 concentration of the surrounding air is artificially elevated. These experiments generally utilize lower CO2 levels than the greenhouse studies. They show lower gains in growth than greenhouse studies, with the gains depending heavily on the species under study. A 2005 review of 12 experiments at 475–600 ppm showed an average gain of 17% in ingather yield, with legumes typically showing a greater response than other species and C4 plants generally showing less. The review likewise stated that the experiments accept their ain limitations. The studied COtwo levels were lower, and most of the experiments were carried out in temperate regions.[73] Satellite measurements establish increasing leaf area alphabetize for 25% to 50% of Earth's vegetated area over the by 35 years (i.e., a greening of the planet), providing evidence for a positive CO2 fertilization outcome.[74] [75]
A 2017 Politico article states that increased CO2 levels may have a negative impact on the nutritional quality of various human food crops, by increasing the levels of carbohydrates, such as glucose, while decreasing the levels of important nutrients such every bit poly peptide, iron, and zinc. Crops experiencing a subtract in protein include rice, wheat, barley and potatoes.[76] [ scientific citation needed ]
Atmospheric CO2 and the oceanic carbon wheel [edit]
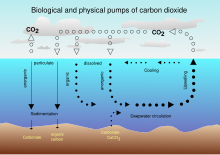
The Earth's oceans contain a large amount of CO2 in the form of bicarbonate and carbonate ions—much more than than the corporeality in the atmosphere. The bicarbonate is produced in reactions between rock, water, and carbon dioxide. I example is the dissolution of calcium carbonate:
- CaCO
3 + COtwo + H
2 O ⇌ Ca 2+
+ two HCO −
iii
Reactions like this tend to buffer changes in atmospheric CO2. Since the right side of the reaction produces an acidic compound, adding CO2 on the left side decreases the pH of seawater, a process which has been termed ocean acidification (pH of the bounding main becomes more acidic although the pH value remains in the alkaline range). Reactions between CO2 and non-carbonate rocks too add bicarbonate to the seas. This tin can later undergo the reverse of the above reaction to grade carbonate rocks, releasing one-half of the bicarbonate every bit CO2. Over hundreds of millions of years, this has produced huge quantities of carbonate rocks.
Ultimately, most of the CO2 emitted by human being activities volition dissolve in the ocean;[77] nevertheless, the charge per unit at which the body of water will have information technology up in the hereafter is less sure. Even if equilibrium is reached, including dissolution of carbonate minerals, the increased concentration of bicarbonate and decreased or unchanged concentration of carbonate ion will requite rising to a higher concentration of united nations-ionized carbonic acid and dissolved CO2. This higher concentration in the seas, forth with higher temperatures, would mean a college equilibrium concentration of CO2 in the air.[78] [79]
Carbon dioxide has unique long-term effects on climatic change that are about "irreversible" for a thousand years after emissions stop (goose egg farther emissions). The greenhouse gases marsh gas and nitrous oxide do not persist over fourth dimension in the same way equally carbon dioxide. Fifty-fifty if human carbon dioxide emissions were to completely cease, atmospheric temperatures are not expected to subtract significantly in the brusk term. This is because the air temperature is determined by a residue between heating, due to greenhouse gases, and cooling due to estrus transfer to the ocean. If emissions were to cease, COii levels and the heating result would slowly decrease, but simultaneously the cooling due to oestrus transfer would diminish (because sea temperatures would go closer to the air temperature), with the issue that the air temperature would decrease just slowly. Sea temperatures would go on to rise, causing thermal expansion and some sea level rise.[78] Lowering global temperatures more speedily would require carbon sequestration or geoengineering.
Carbon moves between the atmosphere, vegetation (dead and alive), the soil, the surface layer of the ocean, and the deep bounding main. A detailed model has been developed by Fortunat Joos in Bern and colleagues, chosen the Bern model.[lxxx] A simpler model based on it gives the fraction of CO2 remaining in the atmosphere as a function of the number of years after information technology is emitted into the atmosphere:[81]
Co-ordinate to this model, 21.7% of the carbon dioxide released into the air stays there forever, but of course this is non true if carbon-containing material is removed from the bicycle (and stored) in means that are not operative now (bogus sequestration).
Anthropogenic COtwo emissions [edit]

The U.s., China and Russian federation have cumulatively contributed the greatest amounts of CO2 since 1850.[82]

While COii absorption and release is always happening as a result of natural processes, the contempo rise in CO2 levels in the temper is known to be mainly due to human (anthropogenic) action.[86] In that location are 4 means human action, especially fossil fuel called-for, is known to have caused the rapid increment in atmospheric COii over the last few centuries:
- Various national statistics bookkeeping for fossil fuel consumption, combined with knowledge of how much atmospheric COtwo is produced per unit of fossil fuel (e.g. liter of gasoline).[87]
- Past examining the ratio of various carbon isotopes in the temper.[86] The burning of long-buried fossil fuels releases CO2 containing carbon of unlike isotopic ratios to those of living plants, enabling stardom between natural and human-caused contributions to CO2 concentration.
- Higher atmospheric COtwo concentrations in the Northern Hemisphere, where most of the world'due south population lives (and emissions originate from), compared to the southern hemisphere. This difference has increased equally anthropogenic emissions have increased.[88]
- Atmospheric Oii levels are decreasing in Earth's atmosphere equally it reacts with the carbon in fossil fuels to form CO2.[89]
Burning fossil fuels such as coal, petroleum, and natural gas is the leading crusade of increased anthropogenic CO2; deforestation is the second major crusade. In 2010, 9.14 gigatonnes of carbon (GtC, equivalent to 33.5 gigatonnes of CO2 or about four.three ppm in Earth's temper) were released from fossil fuels and cement production worldwide, compared to 6.15 GtC in 1990.[90] In add-on, country utilise alter contributed 0.87 GtC in 2010, compared to 1.45 GtC in 1990.[ninety] In 1997, human being-acquired Indonesian peat fires were estimated to take released between 13% and forty% of the average annual global carbon emissions caused past the called-for of fossil fuels.[91] [92] [93] In the period 1751 to 1900, about 12 GtC were released as COii to the temper from called-for of fossil fuels, whereas from 1901 to 2013 the figure was about 380 GtC.[94]
The Integrated Carbon Ascertainment Arrangement (ICOS) continuously releases data about CO2 emissions, budget and concentration at individual observation stations.
| Year | Fossil fuels and industry Gt C | State apply change Gt C | Total Gt C | Total Gt COtwo |
|---|---|---|---|---|
| 2010 | 9.05 | 1.38 | x.43 | 38.2 |
| 2011 | 9.35 | one.34 | 10.69 | 39.2 |
| 2012 | ix.5 | 1.47 | 10.97 | twoscore.three |
| 2013 | 9.54 | 1.52 | 11.06 | 40.6 |
| 2014 | ix.61 | ane.66 | 11.27 | 41.4 |
| 2015 | 9.62 | i.7 | eleven.32 | 41.5 |
| 2016 | nine.66 | 1.54 | 11.two | 41.1 |
| 2017 | 9.77 | 1.47 | eleven.24 | 41.iii |
| 2018 | ix.98 | 1.51 | xi.49 | 42.i |
| 2019 (projection) | ten.0 | one.8 | 11.eight | 43.one |
Anthropogenic carbon emissions exceed the amount that can be taken up or balanced out by natural sinks.[96] As a outcome, carbon dioxide has gradually accumulated in the atmosphere, and as of 2019[update], its concentration is almost 48% above pre-industrial levels.[11] Diverse techniques have been proposed for removing excess carbon dioxide from the temper (run into Carbon sink#Artificial sequestration). Currently about half of the carbon dioxide released from the burning of fossil fuels is not captivated by vegetation and the oceans and remains in the atmosphere.[97]
-

Global fossil carbon emissions 1800–2014
-

False-color image of smoke and ozone pollution from Indonesian fires, 1997
-

Biosphere CO2 flux in the northern hemisphere winter (NOAA Carbon Tracker)
-

Biosphere CO2 flux in the northern hemisphere summer (NOAA Carbon Tracker)
Ongoing measurements of atmospheric CO2 [edit]

Carbon Dioxide observations from 2005 to 2014 showing the seasonal variations and the deviation between northern and southern hemispheres
The commencement reproducibly authentic measurements of atmospheric CO2 were from flask sample measurements made by Dave Keeling at Caltech in the 1950s.[98] A few years later in March 1958 the beginning ongoing measurements were started by Keeling at Mauna Loa. Measurements at Mauna Loa have been ongoing since then. Now measurements are made at many sites globally. Additional measurement techniques are also used likewise. Many measurement sites are role of larger global networks. Global network data are often fabricated publicly available on the weather of proper acquittance according to the respective data user policies.
There are several surface measurement (including flasks and continuous in situ) networks including NOAA/ERSL,[99] WDCGG,[100] and RAMCES.[101] The NOAA/ESRL Baseline Observatory Network, and the Scripps Institution of Oceanography Network[102] data are hosted at the CDIAC at ORNL. The World Information Centre for Greenhouse Gases (WDCGG), part of GAW, information are hosted by the JMA. The Reseau Atmospherique de Mesure des Composes an Effet de Serre database (RAMCES) is office of IPSL.
From these measurements, further products are fabricated which integrate data from the diverse sources. These products likewise address issues such as data discontinuity and sparseness. GLOBALVIEW-CO2 is ane of these products.[103]
Ongoing ground-based total column measurements began more than recently. Column measurements typically refer to an averaged column amount denoted XCO2, rather than a surface only measurement. These measurements are made by the TCCON. These data are likewise hosted on the CDIAC, and fabricated publicly available according to the data utilize policy.[104]
Satellite measurements are also a contempo addition to atmospheric 10CO2 measurements. SCIAMACHY aboard ESA's ENVISAT made global column TenCO2 measurements from 2002 to 2012. Airs aboard NASA'south Aqua satellite makes global TenCO2 measurements and was launched soon after ENVISAT in 2012. More contempo satellites have significantly improved the data density and precision of global measurements. Newer missions have higher spectral and spatial resolutions. JAXA'south GOSAT was the first dedicated GHG monitoring satellite to successfully attain orbit in 2009. NASA's OCO-2 launched in 2014 was the second. Various other satellites missions to mensurate atmospheric XCO2 are planned.
Meet also [edit]
- Azolla event – Hypothetical geoclimatic outcome, 49 1000 yrs ago
- Carbon cycle
- Global temperature record
- Keeling Curve - a graph of the accumulation of carbon dioxide in the Earth'southward atmosphere based on measurements taken in Hawaii
References [edit]
- ^ Petty, Chiliad.W. (2004). "A First Course in Atmospheric Radiation". Eos Transactions. 85 (36): 229–51. Bibcode:2004EOSTr..85..341P. doi:10.1029/2004EO360007.
- ^ a b c Eggleton, Tony (2013). A Brusk Introduction to Climate Change. Cambridge Academy Press. p. 52. ISBN9781107618763.
- ^ Bressan, David. "Carbon-Dioxide In Earth's Temper Spikes To Record Level". Forbes . Retrieved 15 April 2021.
- ^ Zhang, Yi Ge; et al. (28 October 2013). "A 40-million-twelvemonth history of atmospheric CO2". Philosophical Transactions of the Royal Society A. 371 (2001): 20130096. Bibcode:2013RSPTA.37130096Z. doi:10.1098/rsta.2013.0096. PMID 24043869.
- ^ Etheridge, D.M.; L.P. Steele; R.L. Langenfelds; R.J. Francey; J.-Thou. Barnola; V.I. Morgan (1996). "Natural and anthropogenic changes in atmospheric CO2 over the last thousand years from air in Antarctic ice and firn". Journal of Geophysical Research. 101 (D2): 4115–28. Bibcode:1996JGR...101.4115E. doi:10.1029/95JD03410. ISSN 0148-0227.
- ^ Millero, Frank J. (1995). "Thermodynamics of the carbon dioxide organization in the oceans". Geochimica et Cosmochimica Acta. 59 (4): 661–77. Bibcode:1995GeCoA..59..661M. doi:10.1016/0016-7037(94)00354-O.
- ^ Feely, R.A.; et al. (July 2004). "Bear upon of Anthropogenic CO2 on the CaCO3 System in the Oceans". Science. 305 (5682): 362–66. Bibcode:2004Sci...305..362F. doi:10.1126/scientific discipline.1097329. PMID 15256664. S2CID 31054160.
- ^ "IPCC Working Group I". www.climatechange2013.org.
- ^ "Earth'south CO2 Habitation Page". www.co2.earth.
- ^ "Carbon dioxide passes symbolic mark". BBC. ten May 2013. Retrieved 10 May 2013.
- ^ a b "Up-to-engagement weekly average CO2 at Mauna Loa". NOAA. Retrieved 1 June 2019.
- ^ "Greenhouse gas levels pass symbolic 400ppm CO2 milestone". The Guardian. Associated Printing. 1 June 2012. Retrieved 11 May 2013.
- ^ "Conversion Tables". Carbon Dioxide Data Assay Center. Oak Ridge National Laboratory. 18 July 2020. Archived from the original on 27 September 2017. Retrieved 18 July 2020. Alt URL Archived 23 February 2016 at the Wayback Machine
- ^ a b Tans, Pieter. "Trends in Carbon Dioxide". NOAA/ESRL. Retrieved 11 Dec 2009.
- ^ "Just CO2unting..." Archived from the original on 18 February 2010.
- ^ a b "Trends in Atmospheric Carbon Dioxide". World System Enquiry Laboratory. NOAA.
- ^ Vaughan, A (6 May 2015). "Global carbon dioxide levels break 400ppm milestone". The Guardian . Retrieved seven May 2015.
- ^ Dlugokencky, E; Tans, P (half dozen May 2015). "ESRL Global Monitoring Division". Earth System Research Laboratory. NOAA. Retrieved 7 May 2015.
- ^ "Carbon Upkeep 2009 Highlights". globalcarbonproject.org. Archived from the original on sixteen December 2011. Retrieved 2 November 2012.
- ^ a b Rasmussen, Carl Edward. "Atmospheric Carbon Dioxide Growth Charge per unit".
- ^ "Ofttimes Asked Questions". Carbon Dioxide Information Analysis Center (CDIAC). Archived from the original on 17 August 2011. Retrieved 13 June 2007.
- ^ Kunzig, Robert (nine May 2013). "Climate Milestone: Earth's COtwo Level Passes 400 ppm". National Geographic . Retrieved 12 May 2013.
- ^ a b "IPCC: Climate Change 2001: The Scientific Basis" (PDF).
- ^ Zahnle, Thou.; Schaefer, L.; Fegley, B. (2010). "Earth's Earliest Atmospheres". Cold Spring Harbor Perspectives in Biology. 2 (10): a004895. doi:ten.1101/cshperspect.a004895. PMC2944365. PMID 20573713.
- ^ Ward, Peter D.; Brownlee, Donald (2003). The life and expiry of planet Earth. Macmillan. pp. 117–28. ISBN978-0-8050-7512-0.
- ^ Caldeira, Ken; Kasting, James F. (Dec 1992). "The life span of the biosphere revisited". Nature. 360 (6406): 721–23. Bibcode:1992Natur.360..721C. doi:10.1038/360721a0. PMID 11536510. S2CID 4360963.
- ^ Etheridge, D.M.; Steele, L.P.; Langenfelds, R.L.; Francey, R.J.; Barnola, JM; Morgan, VI (June 1998). "Historical CO2 tape derived from a spline fit (20-year cutoff) of the Police force Dome DE08 and DE08-2 water ice cores". Carbon Dioxide Information Analysis Center. Oak Ridge National Laboratory. Archived from the original on 12 July 2012. Retrieved 12 June 2007.
- ^ Amos, J. (4 September 2006). "Deep water ice tells long climate story". BBC News . Retrieved 28 April 2010.
- ^ Hileman B. (November 2005). "Ice Core Record Extended: Analyses of trapped air evidence current CO2 at highest level in 650,000 years". Chemical & Engineering science News. 83 (48): 7. doi:10.1021/cen-v083n048.p007. ISSN 0009-2347.
- ^ Vostok Ice Core Data, ncdc.noaa.gov
- ^ Richerson P.J.; Boyd R.; Bettinger R.L. (July 2001). "Was Agriculture Impossible During The Pleistocene But Mandatory During The Holocene?" (PDF). American Artifact. 66 (three): 387–411. doi:x.2307/2694241. JSTOR 2694241. S2CID 163474968.
- ^ a b c Osborne, C.P.; Beerling, D.J. (2006). "Nature's greenish revolution: the remarkable evolutionary ascension of Civ plants". Philosophical Transactions of the Royal Society B: Biological Sciences. 361 (1465): 173–94. doi:10.1098/rstb.2005.1737. PMC1626541. PMID 16553316.
- ^ a b Witkowski, Caitlyn (28 November 2018). "Molecular fossils from phytoplankton reveal secular Pco2 tendency over the Phanerozoic". Science Advances. 2 (11): eaat4556. Bibcode:2018SciA....4.4556W. doi:ten.1126/sciadv.aat4556. PMC6261654. PMID 30498776.
- ^ "New COii information helps unlock the secrets of Antarctic formation". Physorg.com. 13 September 2009.
- ^ Pagani, Mark; Huber, Matthew; Liu, Zhonghui; Bohaty, Steven Yard.; Henderiks, Jorijntje; Sijp, Willem; Krishnan, Srinath; Deconto, Robert M. (2 December 2011). "Drop in carbon dioxide levels led to polar ice sheet, study finds". Scientific discipline. 334 (6060): 1261–iv. Bibcode:2011Sci...334.1261P. doi:10.1126/science.1203909. PMID 22144622. S2CID 206533232. Retrieved 14 May 2013.
- ^ Wagner, Friederike; Bent Aaby; Henk Visscher (2002). "Rapid atmospheric O
2 changes associated with the 8,200-years-B.P. cooling event". Proc. Natl. Acad. Sci. U.s.a.. 99 (19): 12011–fourteen. Bibcode:2002PNAS...9912011W. doi:ten.1073/pnas.182420699. PMC129389. PMID 12202744. - ^ Indermühle, Andreas; Bernhard Stauffer; Thomas F. Stocker (1999). "Early Holocene Atmospheric COii Concentrations". Scientific discipline. 286 (5446): 1815. doi:10.1126/science.286.5446.1815a.
- ^ Smith, H.J.; 1000 Wahlen; D. Mastroianni (1997). "The COtwo concentration of air trapped in GISP2 ice from the Last Glacial Maximum-Holocene transition". Geophysical Inquiry Letters. 24 (1): i–4. Bibcode:1997GeoRL..24....1S. doi:ten.1029/96GL03700.
- ^ "Annex II Glossary". Intergovernmental Panel on Climatic change. Retrieved 15 October 2010.
- ^ A concise description of the greenhouse event is given in the Intergovernmental Panel on Climate change Fourth Assessment Study, "What is the Greenhouse Upshot?" FAQ i.3 – AR4 WGI Chapter i: Historical Overview of Climate change Science, IPCC Fourth Cess Study, Chapter 1, p. 115: "To residuum the absorbed incoming [solar] free energy, the Earth must, on average, radiate the aforementioned amount of energy dorsum to space. Because the Globe is much colder than the Sunday, it radiates at much longer wavelengths, primarily in the infrared office of the spectrum (meet Figure 1). Much of this thermal radiation emitted past the state and ocean is captivated past the atmosphere, including clouds, and reradiated dorsum to World. This is chosen the greenhouse result."
Stephen H. Schneider, in Geosphere-biosphere Interactions and Climate, Lennart O. Bengtsson and Claus U. Hammer, eds., Cambridge University Press, 2001, ISBN 0-521-78238-4, pp. 90–91.
E. Claussen, V.A. Cochran, and D.P. Davis, Climate change: Scientific discipline, Strategies, & Solutions, University of Michigan, 2001. p. 373.
A. Allaby and M. Allaby, A Dictionary of Globe Sciences, Oxford University Press, 1999, ISBN 0-19-280079-five, p. 244. - ^ Vaclav Smil (2003). The Globe's Biosphere: Evolution, Dynamics, and Change. MIT Press. p. 107. ISBN978-0-262-69298-4.
- ^ "Solar Radiation and the Earth'due south Energy Balance". The Climate System – EESC 2100 Spring 2007. Columbia Academy. Archived from the original on 4 November 2004. Retrieved 15 October 2010.
- ^ Le Treut H, Somerville R, Cubasch U, Ding Y, Mauritzen C, Mokssit A, Peterson T, Prather Thousand (2007). "Historical Overview of Climatic change Scientific discipline" (PDF). In Solomon S, Qin D, Manning Chiliad, Chen Z, Marquis M, Averyt KB, Tignor Chiliad, Miller HL (eds.). Climate change 2007: The Physical Scientific discipline Basis. Contribution of Working Group I to the 4th Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, Britain and New York, NY: Cambridge Academy Press. p. 97. Archived from the original (PDF) on 26 November 2018. Retrieved 25 March 2014.
- ^ "The Elusive Accented Surface Air Temperature (SAT)". Goddard Establish for Infinite Studies. NOAA.
- ^ Walker, James C.G. (June 1985). "Carbon dioxide on the early globe" (PDF). Origins of Life and Evolution of the Biosphere. 16 (2): 117–27. Bibcode:1985OrLi...16..117W. doi:10.1007/BF01809466. hdl:2027.42/43349. PMID 11542014. S2CID 206804461. Retrieved thirty January 2010.
- ^ Pavlov, Alexander A.; Kasting, James F.; Brown, Lisa L.; Rages, Kathy A.; Freedman, Richard (May 2000). "Greenhouse warming past CH4 in the atmosphere of early Earth". Journal of Geophysical Research. 105 (E5): 11981–90. Bibcode:2000JGR...10511981P. doi:10.1029/1999JE001134. PMID 11543544.
- ^ a b IPCC Fifth Cess Report – Chapter 8: Anthropogenic and Natural Radiative Forcing.
- ^ Arrhenius, Svante (1896). "On the influence of carbonic acid in the air upon the temperature of the basis" (PDF). Philosophical Magazine and Periodical of Science: 237–76.
- ^ Riebeek, Holli (sixteen June 2011). "The Carbon Cycle". World Observatory. NASA. Archived from the original on v March 2016. Retrieved 5 April 2018.
- ^ a b c Friedlingstein, P., Jones, Thousand., O'Sullivan, G., Andrew, R., Hauck, J., Peters, G., Peters, W., Pongratz, J., Sitch, S., Le Quéré, C. and 66 others (2019) "Global carbon budget 2019". Globe Organisation Scientific discipline Data, xi(4): 1783–1838. doi:10.5194/essd-11-1783-2019.
 Material was copied from this source, which is bachelor under a Artistic Commons Attribution 4.0 International License.
Material was copied from this source, which is bachelor under a Artistic Commons Attribution 4.0 International License. - ^ Kayler, Z., Janowiak, M., Swanston, C. (2017). "The Global Carbon Cycle". Considering Wood and Grassland Carbon in Land Management (PDF). General Technical Written report WTO-GTR-95. United States Department of Agronomics, Forest Service. pp. 3–ix.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ Gerlach, T.One thousand. (4 June 1991). "Present-mean solar day CO2 emissions from volcanoes". Eos, Transactions, American Geophysical Marriage. 72 (23): 249, 254–55. Bibcode:1991EOSTr..72..249.. doi:ten.1029/90EO10192.
- ^ Dlugokencky, Eastward. (5 Feb 2016). "Annual Mean Carbon Dioxide Information". Earth System Research Laboratory. NOAA. Retrieved 12 February 2016.
- ^ Cappelluti, Thousand.; Bösch, H.; Monks, P.S. (2009). Use of remote sensing techniques for the detection and monitoring of GHG emissions from the Scottish country use sector. Scottish Government. ISBN978-0-7559-7738-3.
- ^ a b c Junling Huang; Michael B. McElroy (2012). "The Contemporary and Historical Upkeep of Atmospheric CO2" (PDF). Canadian Journal of Physics. xc (8): 707–xvi. Bibcode:2012CaJPh..ninety..707H. doi:x.1139/p2012-033.
- ^ Canadell JG, Le Quéré C, Raupach MR, et al. (November 2007). "Contributions to accelerating atmospheric CO2 growth from economic activity, carbon intensity, and efficiency of natural sinks". Proc. Natl. Acad. Sci. The statesA. 104 (47): 18866–seventy. Bibcode:2007PNAS..10418866C. doi:10.1073/pnas.0702737104. PMC2141868. PMID 17962418.
- ^ Post WM, Rex AW, Wullschleger SD, Hoffman FM (June 1997). "Historical Variations in Terrestrial Biospheric Carbon Storage". DOE Research Summary. 34 (1): 99–109. Bibcode:1997GBioC..11...99P. doi:x.1029/96GB03942.
- ^ "Written report of the Ocean Acidification and Oxygen Working Grouping, SCOR Biological Observatories Workshop" (PDF). scor-int.org/. International Council for Science'south Scientific Committee on Sea Research (SCOR). thirty September 2009.
- ^ Olson JM (May 2006). "Photosynthesis in the Archean era". Photosyn. Res. 88 (ii): 109–17. doi:x.1007/s11120-006-9040-5. PMID 16453059. S2CID 20364747.
- ^ Buick R (August 2008). "When did oxygenic photosynthesis evolve?". Philos. Trans. R. Soc. Lond. B Biol. Sci. 363 (1504): 2731–43. doi:10.1098/rstb.2008.0041. PMC2606769. PMID 18468984.
- ^ Lovelock, J. E. (1972). "Gaia equally seen through the atmosphere". Atmospheric Environment. 6 (eight): 579–580. Bibcode:1972AtmEn...half-dozen..579L. doi:ten.1016/0004-6981(72)90076-5. Archived from the original on 3 November 2011. Retrieved 22 March 2014.
- ^ Li, K.-F. (thirty May 2009). "Atmospheric pressure level as a natural climate regulator for a terrestrial planet with a biosphere". Proceedings of the National Academy of Sciences. 106 (24): 9576–9579. Bibcode:2009PNAS..106.9576L. doi:10.1073/pnas.0809436106. PMC2701016. PMID 19487662. Retrieved 22 March 2014.
- ^ Nealson KH, Conrad PG (December 1999). "Life: by, present and future". Philos. Trans. R. Soc. Lond. B Biol. Sci. 354 (1392): 1923–39. doi:10.1098/rstb.1999.0532. PMC1692713. PMID 10670014.
- ^ Whitmarsh J, Govindjee (1999). "The photosynthetic process". In Singhal GS; Renger M; Sopory SK; Irrgang KD; Govindjee (eds.). Concepts in photobiology: photosynthesis and photomorphogenesis. Boston: Kluwer Academic Publishers. pp. 11–51. ISBN978-0-7923-5519-nine.
100 10 1015 grams of carbon/year fixed past photosynthetic organisms which is equivalent to iv x x18 kJ/yr = four ten 1021J/yr of free free energy stored as reduced carbon; (iv x 1018 kJ/yr) / (31,556,900 sec/yr) = 1.27 x xxiv J/twelvemonth; (1.27 x 10fourteen J/yr) / (1012 J/sec / TW) = 127 TW.
- ^ Steger U, Achterberg W, Blok Chiliad, Bode H, Frenz Westward, Gather C, Hanekamp G, Imboden D, Jahnke M, Kost M, Kurz R, Nutzinger HG, Ziesemer T (2005). Sustainable development and innovation in the energy sector. Berlin: Springer. p. 32. ISBN978-three-540-23103-v.
The average global charge per unit of photosynthesis is 130 TW (1 TW = 1 terawatt = 1012 watt).
- ^ "World Consumption of Chief Energy by Energy Blazon and Selected Country Groups, 1980–2004". Energy Information Assistants. 31 July 2006. Archived from the original (XLS) on 9 November 2006. Retrieved 2007-01-20 .
- ^ Field CB, Behrenfeld MJ, Randerson JT, Falkowski P (July 1998). "Principal production of the biosphere: integrating terrestrial and oceanic components". Science. 281 (5374): 237–40. Bibcode:1998Sci...281..237F. doi:10.1126/science.281.5374.237. PMID 9657713.
- ^ "Photosynthesis". McGraw-Colina Encyclopedia of Scientific discipline & Technology. Vol. 13. New York: McGraw-Colina. 2007. ISBN978-0-07-144143-viii.
- ^ Bryant DA, Frigaard NU (November 2006). "Prokaryotic photosynthesis and phototrophy illuminated". Trends Microbiol. xiv (11): 488–96. doi:10.1016/j.tim.2006.09.001. PMID 16997562.
- ^ "Scientists discover unique microbe in California's largest lake". Retrieved 20 July 2009.
- ^ Poorter, Hendrik. "Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration" (PDF).
- ^ Wong, S.C. (December 1979). "Elevated Partial Pressure level of CO2 and Found Growth". Oecologia. 44 (1): 68–74. Bibcode:1979Oecol..44...68W. doi:x.1007/BF00346400. PMID 28310466. S2CID 24541633.
- ^ Ainsworth, Lisa (February 2005). "What accept we learned from xv years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy backdrop and plant production to rise CO2". New Phytol. 165 (2): 351–71. doi:10.1111/j.1469-8137.2004.01224.x. PMID 15720649.
- ^ Zhu, Zaichun; Piao, Shilong; Myneni, Ranga B.; Huang, Mengtian; Zeng, Zhenzhong; Canadell, Josep G.; Ciais, Philippe; Sitch, Stephen; Friedlingstein, Pierre (August 2016). "Greening of the Earth and its drivers". Nature Climatic change. half-dozen (viii): 791–95. Bibcode:2016NatCC...vi..791Z. doi:10.1038/nclimate3004. ISSN 1758-6798.
Nosotros testify a persistent and widespread increase of growing flavor integrated LAI (greening) over 25% to 50% of the global vegetated area, whereas less than iv% of the world shows decreasing LAI (browning). Factorial simulations with multiple global ecosystem models suggest that CO2 fertilization effects explain 70% of the observed greening trend
- ^ Hille, Karl (25 April 2016). "Carbon Dioxide Fertilization Greening Globe, Study Finds". NASA . Retrieved 4 February 2018.
- ^ Evich, Helena Bottemiller; Johnson, Geoff (xiii September 2017). "The great food plummet. The atmosphere is literally changing the food we eat, for the worse. And almost nobody is paying attending". Political leader - The Calendar . Retrieved 22 September 2017.
- ^ Archer, D. (2005). "Fate of fossil fuel COtwo in geologic time". J. Geophys. Res. 110. Bibcode:2005JGRC..11009S05A. doi:10.1029/2004JC002625.
- ^ a b Susan Solomon; Gian-Kasper Plattner; Reto Knutti; Pierre Friedlingstein (February 2009). "Irreversible climate change due to carbon dioxide emissions". Proc. Natl. Acad. Sci. United states. 106 (vi): 1704–09. Bibcode:2009PNAS..106.1704S. doi:10.1073/pnas.0812721106. PMC2632717. PMID 19179281.
- ^ Archer, David; Eby, Michael; Brovkin, Victor; Ridgwell, Andy; Cao, Long; Mikolajewicz, Uwe; Caldeira, Ken; Matsumoto, Katsumi; Munhoven, Guy; Montenegro, Alvaro; Tokos, Kathy (2009). "Atmospheric Lifetime of Fossil Fuel Carbon Dioxide". Annual Review of Earth and Planetary Sciences. 37 (1): 117–34. Bibcode:2009AREPS..37..117A. doi:ten.1146/annurev.earth.031208.100206. hdl:2268/12933. ISSN 0084-6597.
- ^ Fortunat Joos; et al. (December 2001). "Global warming feedbacks on terrestrial carbon uptake under the Intergovernmental Panel on Climate Change (IPCC) Emission Scenarios". Global Biogeochemical Cycles. 15 (4): 891–907. Bibcode:2001GBioC..15..891J. doi:10.1029/2000GB001375.
- ^ Morgan Edwards & Jessika Trancik (25 April 2014). "Supplementary Data" (PDF). Nature Climate Change. doi:x.1038/nclimate2204. hdl:1721.1/96138. , supplement to Climate impacts of free energy technologies depend on emissions timing
- ^ Evans, Simon (5 October 2021). "Analysis: Which countries are historically responsible for climate modify? / Historical responsibility for climatic change is at the heart of debates over climate justice". CarbonBrief.org. Carbon Brief. Archived from the original on 26 Oct 2021.
Source: Carbon Brief analysis of figures from the Global Carbon Projection, CDIAC, Our World in Data, Carbon Monitor, Houghton and Nassikas (2017) and Hansis et al (2015).
- ^ Buis, Alan; Ramsayer, Kate; Rasmussen, Ballad (12 November 2015). "A Breathing Planet, Off Rest". NASA . Retrieved thirteen November 2015.
- ^ Staff (12 November 2015). "Audio (66:01) - NASA News Briefing – Carbon & Climate Telecon". NASA . Retrieved 12 November 2015.
- ^ St. Fleur, Nicholas (10 November 2015). "Atmospheric Greenhouse Gas Levels Hit Record, Study Says". The New York Times . Retrieved xi November 2015.
- ^ a b eastward.g. Gosh, Prosenjit; Brand, Willi A. (2003). "Stable isotope ratio mass spectrometry in global climate change research" (PDF). International Journal of Mass Spectrometry. 228 (ane): 1–33. Bibcode:2003IJMSp.228....1G. CiteSeerX10.i.one.173.2083. doi:10.1016/S1387-3806(03)00289-half-dozen.
Global modify issues have become meaning due to the sustained rise in atmospheric trace gas concentrations (CO2, N
2 O, CH
4 ) over recent years, attributable to the increased per capita energy consumption of a growing global population. - ^ Mohr, Southward.H.; Wang, J.; Ellem, G.; Ward, J.; Giurco, D. (1 Feb 2015). "Projection of world fossil fuels by country". Fuel. 141: 120–135. doi:10.1016/j.fuel.2014.ten.030. Retrieved 19 November 2016.
- ^ Keeling, Charles D.; Piper, Stephen C.; Whorf, Timothy P.; Keeling, Ralph F. (2011). "Evolution of natural and anthropogenic fluxes of atmospheric CO2 from 1957 to 2003". Tellus B. 63 (1): 1–22. Bibcode:2011TellB..63....1K. doi:10.1111/j.1600-0889.2010.00507.x. ISSN 0280-6509.
- ^ Bender, Michael Fifty.; Ho, David T.; Hendricks, Melissa B.; Mika, Robert; Battle, Marker O.; Tans, Pieter P.; Conway, Thomas J.; Sturtevant, Blake; Cassar, Nicolas (2005). "Atmospheric O2/N2changes, 1993–2002: Implications for the partitioning of fossil fuel CO2sequestration". Global Biogeochemical Cycles. 19 (iv): n/a. Bibcode:2005GBioC..19.4017B. doi:10.1029/2004GB002410. ISSN 0886-6236.
- ^ a b "Global carbon budget 2010 (summary)". Tyndall Eye for Climate Change Research. Archived from the original on 23 July 2012.
- ^ Folio, S.; Siegert, F.; Rieley, J.; Boehm, H.; Jaya, A.; Limin, S. (2002). "The amount of carbon released from peat and wood fires in Indonesia during 1997". Nature. 420 (6911): 61–65. Bibcode:2002Natur.420...61P. doi:ten.1038/nature01131. PMID 12422213. S2CID 4379529.
- ^ Lazaroff, True cat (8 November 2002). "Indonesian Wildfires Accelerated Global Warming". Surround New Service. Archived from the original on 8 September 2019. Retrieved 7 November 2011.
- ^ Pearce, Fred (6 Nov 2004). "Massive peat fire is speeding climate change". New Scientist.
- ^ Calculated from file global.1751_2013.csv in [1] Archived 22 October 2011 at the Wayback Auto from the Carbon Dioxide Information Analysis Center.
- ^ "Global Carbon Budget 2019". ICOS. Retrieved 26 January 2020.
- ^ Ballantyne, A.P.; Alden, C.B.; Miller, J.B.; Tans, P.P.; White, J.Westward.C. (2012). "Increase in observed net carbon dioxide uptake by country and oceans during the past 50 years". Nature. 488 (7409): lxx–72. Bibcode:2012Natur.488...70B. doi:x.1038/nature11299. ISSN 0028-0836. PMID 22859203. S2CID 4335259.
- ^ A.P. Ballantyne; C.B. Alden; J.B. Miller; P.P. Tans; J.W. C. White (2012). "Increase in observed net carbon dioxide uptake by land and oceans during the past 50 years". Nature. 488 (7409): 70–72. Bibcode:2012Natur.488...70B. doi:10.1038/nature11299. PMID 22859203. S2CID 4335259.
- ^ "The Early on Keeling Bend | Scripps CO2 Program". scrippsco2.ucsd.edu.
- ^ NOAA CCGG folio Retrieved 2 March 2016
- ^ WDCGG webpage Archived 6 Apr 2016 at the Wayback Automobile Retrieved 2 March 2016
- ^ RAMCES webpage [ permanent expressionless link ] Retrieved 2 March 2016
- ^ CDIAC CO2 page Retrieved nine February 2016
- ^ GLOBALVIEW-CO2 information folio. Retrieved 9 February 2016
- ^ TCCON data utilise policy webpage Retrieved ix February 2016
External links [edit]
- Electric current global map of carbon dioxide concentrations.
- Global Carbon Dioxide Circulation (NASA; thirteen December 2016)
- Video (03:ten) – A Yr in the Life of World's CO2 (NASA; 17 Nov 2014)
Source: https://en.wikipedia.org/wiki/Carbon_dioxide_in_Earth%27s_atmosphere
Posted by: fosdickgagainfoute.blogspot.com


0 Response to "How To Change A 28 Spline To A 31"
Post a Comment